Fluorescence Lifetime Imaging Module
• Modular upgrade for existing microscopes
• Time-gated FLIM approach
• Lifetime discrimination <100ps
Product Description
Based on open source technology developed by Paul French’s team at Imperial College London and using the latest generation gated optical image intensifier from Kentech Instruments Ltd, this high speed time-gated fluorescence lifetime imaging module has been applied to FLIM microscopy, high content analysis and optical FLIM tomography.
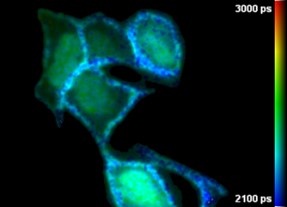
The Cairn FLIM module adds the ability to discriminate signals from fluorescent probes that have overlapping emission profiles that cannot be separated using their spectral intensity alone, to quantify FRET measurements, including signals from genetically expressed FRET biosensors, and to quantify cellular and tissue autofluorescence signals. Running at frame rates up to 10 Hz (depending on sample brightness), this time-gated FLIM approach can discriminate lifetime differences of less than 100 ps with a spatial resolution of 20 line pairs/mm. (Sparks et al, Rev. Sci. Instrum., 2017)
Time-gated FLIM can be realised by adding our FLIM module to existing microscopes with appropriate ultrafast excitation source facilities or can be provided as part of a complete standalone FLIM microscope. If you are interested in adding this modality to your capabilities, please contact us for further information.
Get a Quote
Technical Description
Exemplar FLIM instruments
Wide-field time-gated fluorescence lifetime imaging microscope
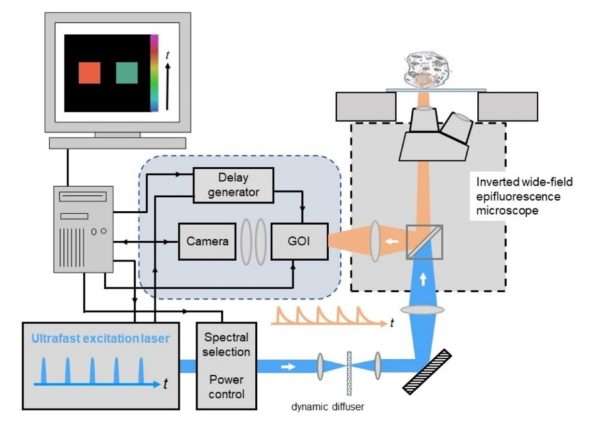
The schematic below shows an overview of the components used for wide-field time-gated FLIM comprising the core elements : (i) ultrafast laser excitation source, (ii) Gated Optical Intensifier (GOI) and computer-controlled delay generator, (iii) computer for data acquisition and analysis, and (iv) a wide-field epifluorescence microscope with cooled readout camera.
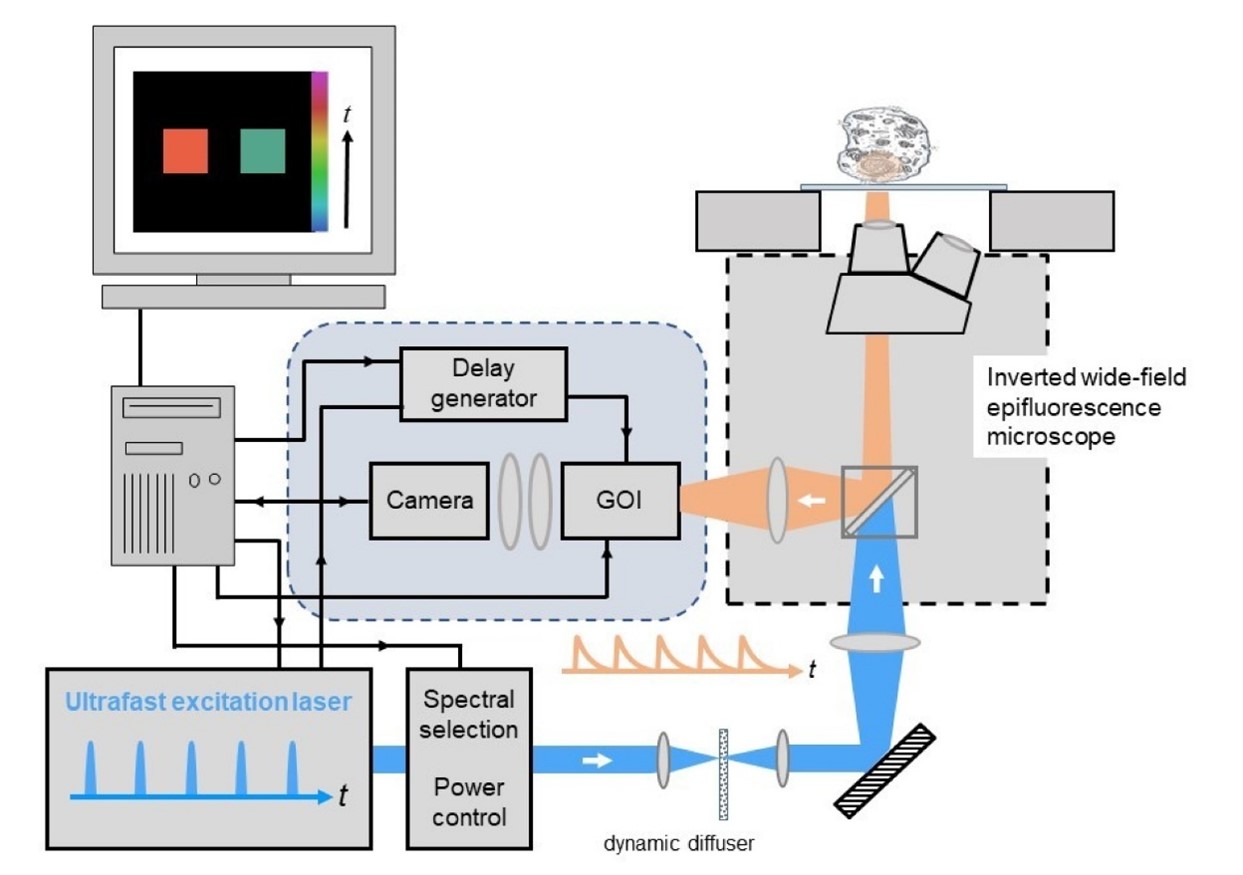
image: Wide-field time-gated FLIM
Wide-field optically sectioned time-gated fluorescence lifetime imaging microscope
For optically sectioned FLIM, the wide-field time-gated FLIM module can be integrated with optical sectioning using a Nipkow spinning disc scanner to provide quasi-confocal FLIM at frame rates up to 10Hz. This is particularly useful for quantitative live cell FLIM since the high degree of parallelisation (e.g. compared to laser scanning microscopy) greatly reduces the instantaneous light dose at the sample and so reduces photobleaching and phototoxicity. The instrument configuration is similar to that for widefield time-gated FLIM except that the excitation light is coupled to the spinning disc scanner via a polarisation-preserving single mode optical fibre, as depicted below, and the FLIM module is mounted at the camera port of the Nipkow disc scanning unit.
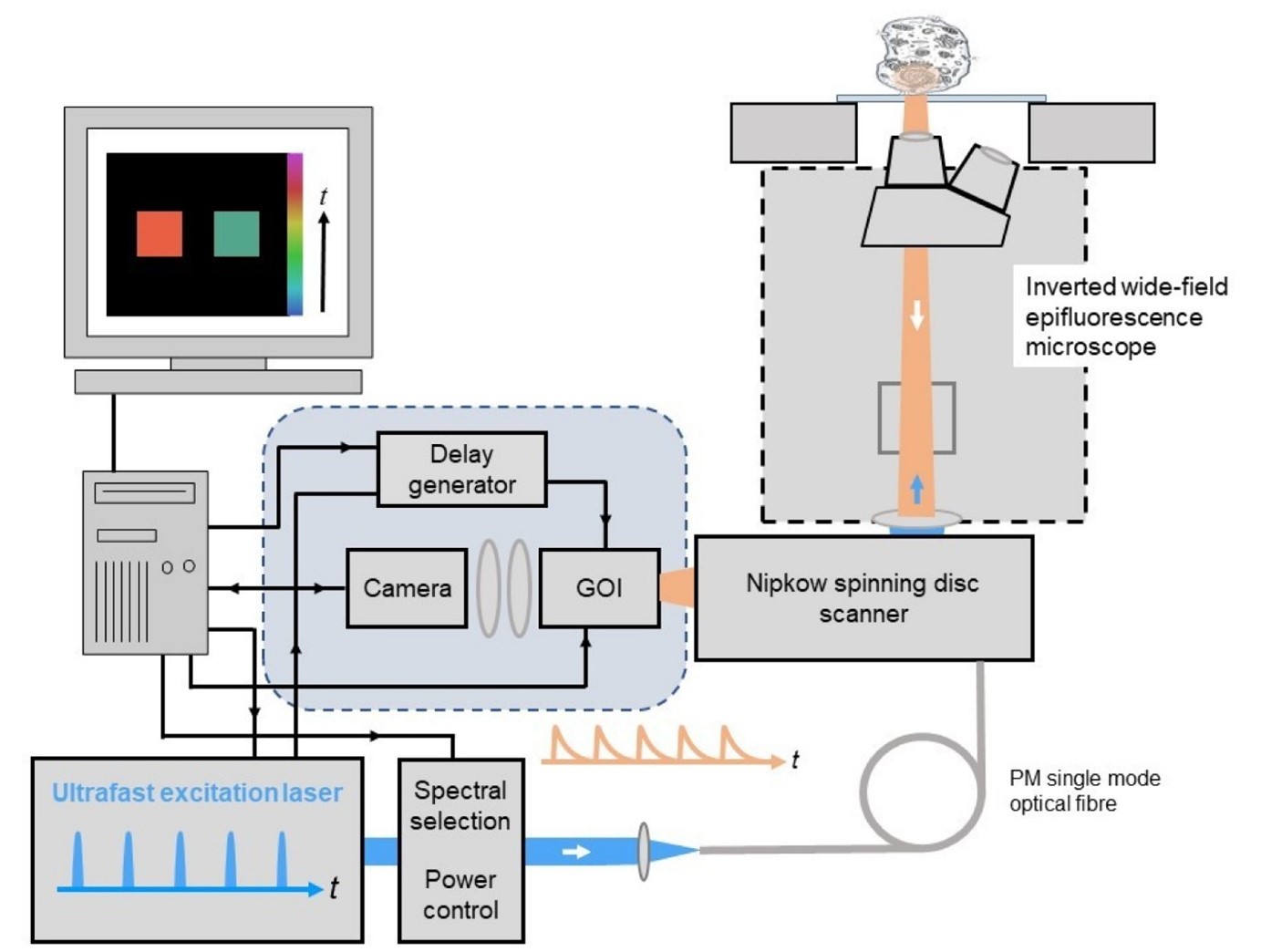
image: Optical sectioned time-gated FLIM
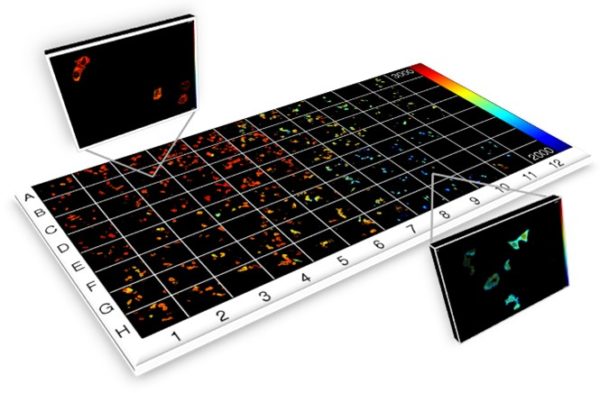
Both of these approaches can be implemented on fully motorised microscopes to provide automated multiwell plate imaging for FLIM high content analysis. Wide-field time-gated FLIM can also be combined with hyperspectral imaging to implement fluorescence lifetime resolved hyperspectral imaging and combined with polarisation-resolved imaging to realise time-resolved anisotropy imaging.
Wide-field time-gated FLIM has been applied to study protein interactions in cell-based studies and to tissue autofluorescence to explore label-free diagnosis of disease, including cancer.
openFLIM software
The system is configured with FLIM data acquisition achieved using the open source software, openFLIM-GOI, which is a MicroManager plug-in shared by Paul French’s team. This enables calibration and configuration of the system for specific experiments and saves the time-gated FLIM data as OME.TIFF. openFLIM-GOI was developed at Imperial College London. If you wish to write your own FLIM software, we can provide a DLL for MicroManager or LabView.
FLIM data analysis may be undertaken using FLIMfit, an open source MATLAB-based software tool that provides an unrivalled range of fitting techniques including global fitting of regions of interest and global analysis.
Citations
Exemplar publications using openFLIM technology
K. Dowling, M. Dayel, M. Lever, P. French, J. Hares, and A. Dymoke-Bradshaw, “Fluorescence lifetime imaging with picosecond resolution for biomedical applications,” Opt. Lett. 23, 810-812 (1998);
D. S. Elson, J. Siegel, S. E. D. Webb, S. Lévêque-Fort, M. J. Lever, P. M. W. French, K. Lauritsen, M. Wahl, R. Erdmann, “ Fluorescence lifetime system for microscopy and multi-well plate imaging using a blue picosecond diode laser”, Opt Lett, 27 (2002) 1409-1411;
D. Grant, J. McGinty, E.J. McGhee, T.D. Bunney, D.M. Owen, C.B. Talbot, W. Zhang, S. Kumar, I. Munro, P. Lanigan, G. Kennedy, C. Dunsby, A.I. Magee, P. Courtney, M. Katan, M.A.A. Neil & P.M.W. French, “High speed optically sectioned fluorescence lifetime imaging permits study of live cell signaling events”, Opt. Expr.15 (2007) 15656 – 15673;
D. M. Owen, E. Auksorius, H. B. Manning, C. B. Talbot, P. A.A. de Beule, M. A. A. Neil and P. M. W. French, “Excitation-resolved hyperspectral fluorescence lifetime imaging using a UV-extended supercontinuum source”, Opt. Lett. 32 (2007) 3408-3410;
Richard K. P. Benninger, Bjorn Önfelt, Oliver Hofmann, Daniel M. Davis, Mark A. A. Neil, Paul M. W. French and Andrew J. DeMello, “Fluorescence‐Lifetime Imaging of DNA–Dye Interactions within Continuous‐Flow Microfluidic Systems”, Angewandte Chemie International Edition, 46 (2007) 2228-2231;
D. M. Grant, W. Zhang, E. J. McGhee, T. D. Bunney, C. B. Talbot, S. Kumar, I Munro, C. Dunsby, M. A. A. Neil, M. Katan and P. M. W. French, “Multiplexed FRET to monitor multiple signalling events in live cells”, Biophysical Journal: Biophysical Letters 95 (2008) L69-L71;
J. McGinty, N. P Galletly, C. Dunsby, I. Munro, D. S Elson, J. Requejo-Isidro, P. Cohen, R. Ahmad, A Forsyth, A. V Thillainayagam, M. A. A. Neil, P. M W French and G.W Stamp, “Wide-field fluorescence lifetime imaging of cancer”, Biomedical Optics Express, 1 (2010) 627-640;
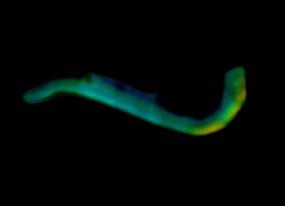
J. McGinty, H. Taylor, L. Chen, L. Bugeon, J. Lamb, M. Dallman, and P. French, “In vivo fluorescence lifetime optical projection tomography,” Biomed. Opt. Express 2, 1340-1350 (2011)
D. Alibhai, D. J. Kelly, S. Warren, S. Kumar, A. Margineanu, R. A. Serwa, E. Thinon, Y. Alexandrov, E. J. Murray, F. Stuhmeier, E. W. Tate, M. A.A. Neil, C. Dunsby and P. M.W. French, “Automated fluorescence lifetime imaging plate reader and its application to Förster resonant energy transfer readout of Gag protein aggregation”, J. Biophotonics 6 (2012) 398-408,
D. J. Kelly, S. C. Warren, S. Kumar, J. L. Lagarto, B T. Dyer, A. Margineanu1, E. W.-F. Lam, C. Dunsby and P. M. W. French, “An automated multiwell plate reading FLIM microscope for live cell autofluorescence lifetime assays”, J. Innov. Opt. Health Sci.. 7 (2014) 1450025-15 pages,
Hugh Sparks, Sean Warren, Joana Guedes, Nagisa Yoshida , Nadia Guerra, Taran Tatla, Chris Dunsby, Paul French, “A flexible wide-field FLIM endoscope utilising blue excitation light for label-free contrast of tissue”, J Biophotonics 8 (2015) 168–178 /
D. J. Kelly, S. C. Warren, D. Alibhai, S. Kumar, Y. Alexandrov, I. Munro, A. Margineanu, J. McCormack, N. J. Welsh, R. A. Serwa, E. Thinon, M. Kongsema, J. McGinty, C. Talbot, E. J. Murray, F. Stuhmeier, M. A.A. Neil, E. W. Tate, V. M. M. Braga, E. W.-F. Lam, C. Dunsby and P. M.W. French, “Automated multiwell fluorescence lifetime imaging for Förster resonant energy transfer assays and High Content Analysis”, Analytical Methods, 7 (2015), 4071-4089,
N. Andrews, M-C Ramel, S. Kumar, Y. Alexandrov, D. J. Kelly, S. C. Warren, L. Kerry, N. Lockwood, A. Frolov, P. Frankel, L. Bugeon, J. McGinty, M. J. Dallman* and P. M. W. French*, “Visualising apoptosis in live zebrafish using fluorescence lifetime imaging with optical projection tomography to map FRET biosensor activity in space and time”, J. Biophoton, 9 (2016) 414–424.
F. Görlitz*, D. J. Kelly*, S. C. Warren, D. Alibhai, L. West, S. Kumar, Y. Alexandrov, I. Munro, J. McGinty, C. Talbot, R. A. Serwa, E. Thinon, V. da Paola, E. J. Murray, F. Stuhmeier, M. A. A. Neil, E. W. Tate, C. Dunsby and P. M. W. French, “Open Source High Content Analysis Utilizing Automated Fluorescence Lifetime Imaging Microscopy”, J. Vis. Exp. 119, (2017) e55119,
H. Sparks*, F. Görlitz*, D.J. Kelly, S.C. Warren, P.A. Kellett, E. Garcia, A.K.L. Dymoke-Bradshaw, J.D. Hares, M. A.A. Neil, C. Dunsby, P.M.W. French, “Characterisation of new gated optical image intensifiers for fluorescence lifetime imaging”, Rev Sci Instrum. 88 (2017) 013707.
Frederik Görlitz, David S. Corcoran, Edwin A. Garcia Castano, Birgit Leitinger, Mark A. A. Neil, Christopher Dunsby and Paul M. W. French, “Mapping Molecular Function to Biological Nanostructure: Combining Structured Illumination Microscopy with Fluorescence Lifetime Imaging (SIM + FLIM)”, Photonics 4 (2017) 40;
Wenjun Guo, Sunil Kumar, Frederik Görlitz, Edwin Garcia, Yuriy Alexandrov, Ian Munro, Douglas J. Kelly, Sean Warren, Peter Thorpe, Christopher Dunsby*, Paul French*, “Automated FLIM HCA of protein-protein interactions between endogenously-labelled kinetochore proteins in live budding yeast cells”, SLAS Technology, 24 (2019) 308-320;